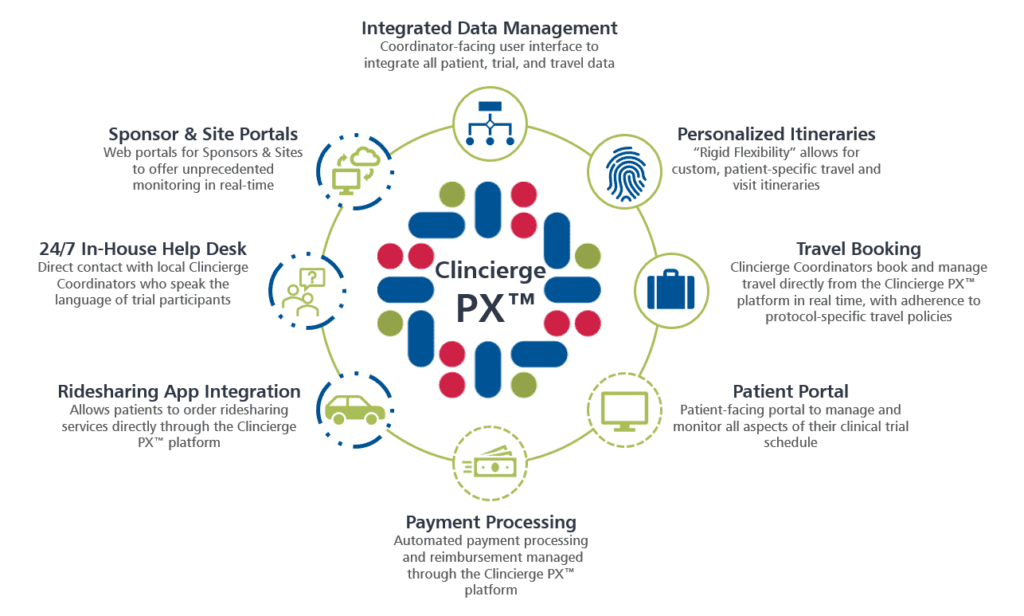
VIDA Launches VIDA Intelligence Portal 2.0 to Accelerate Onboarding of Clinical Trial Sites | Imaging Technology News

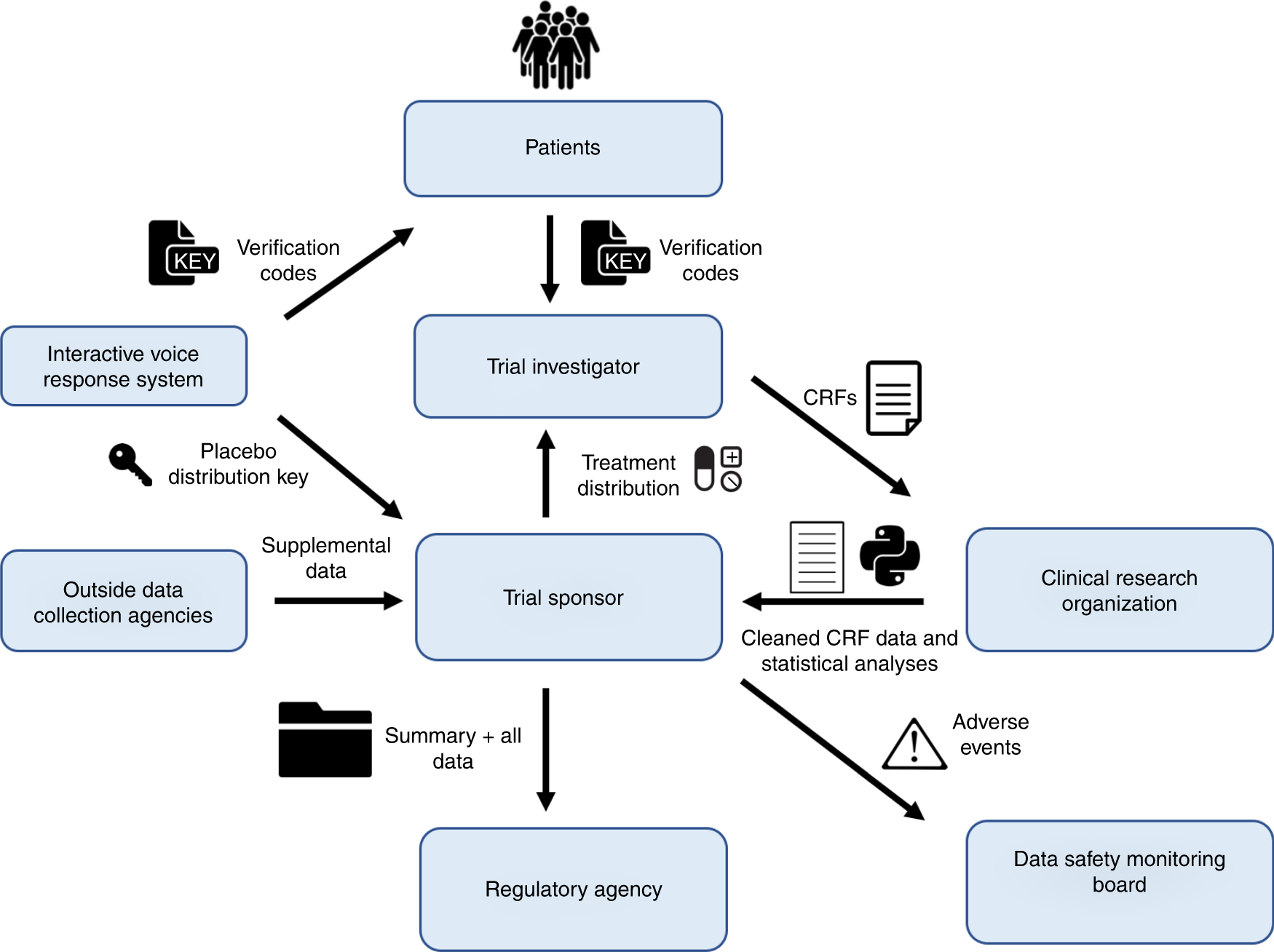
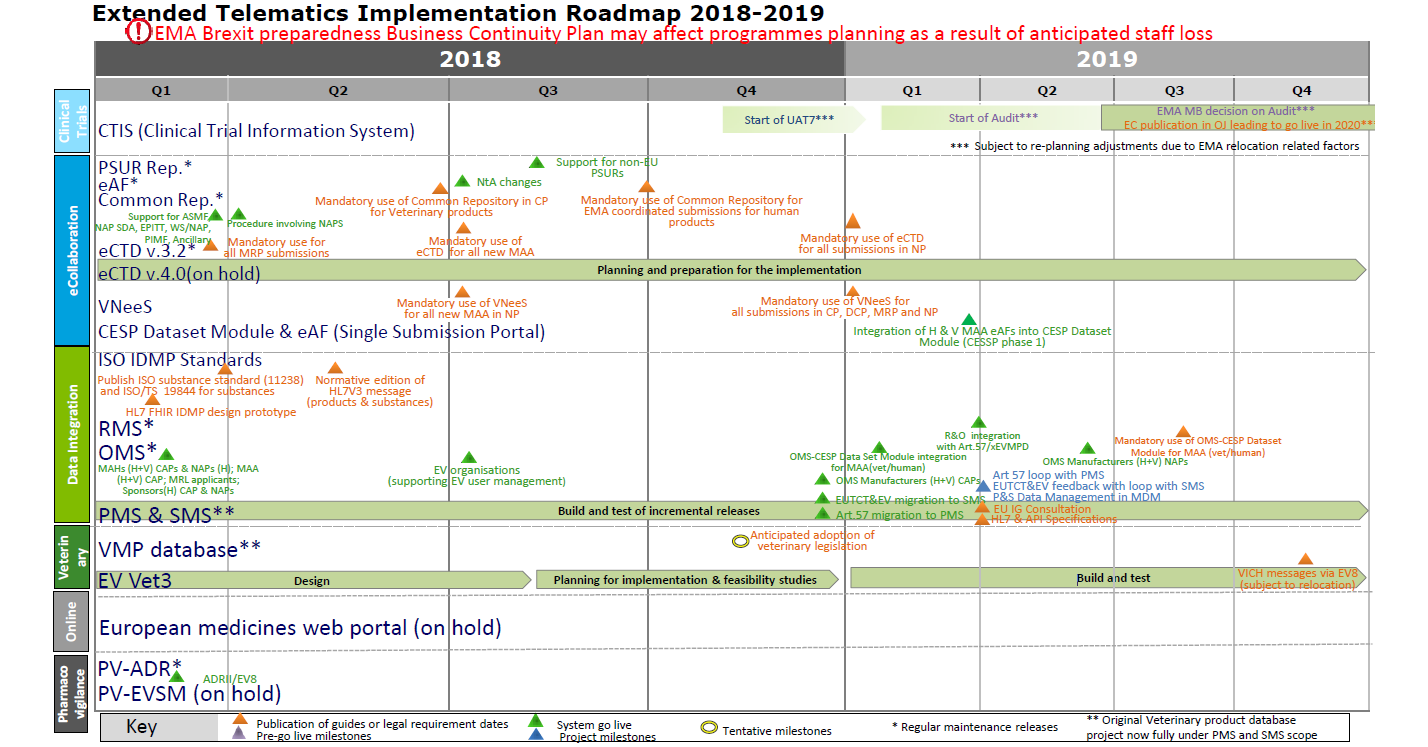
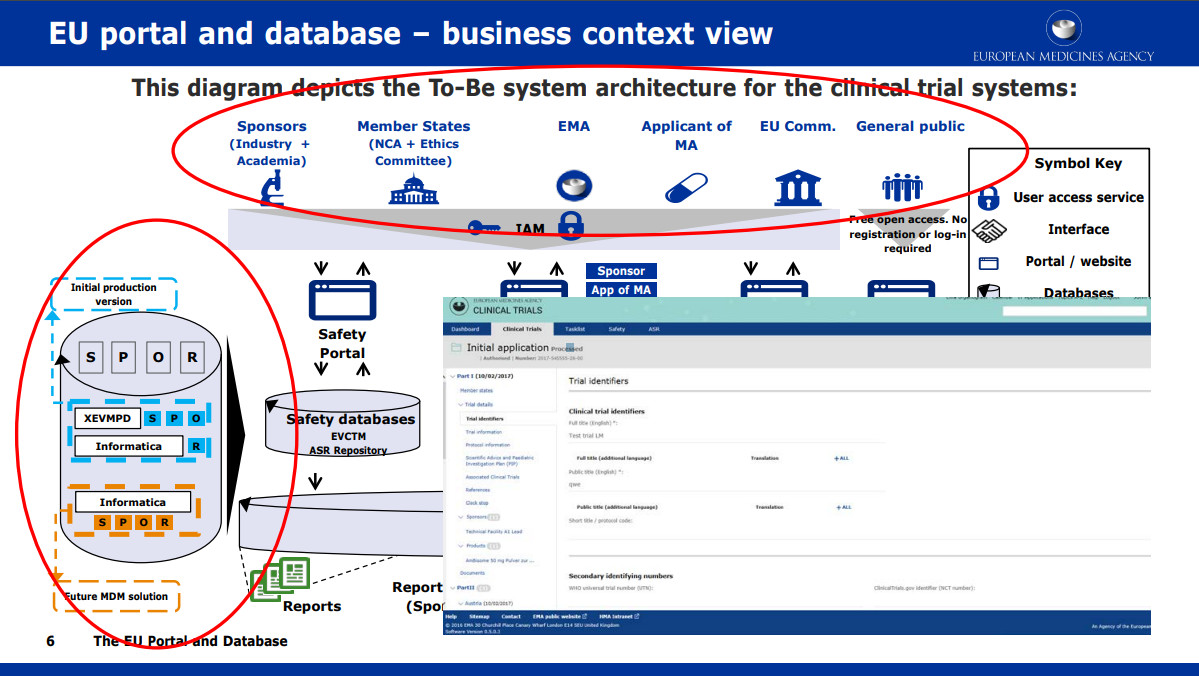
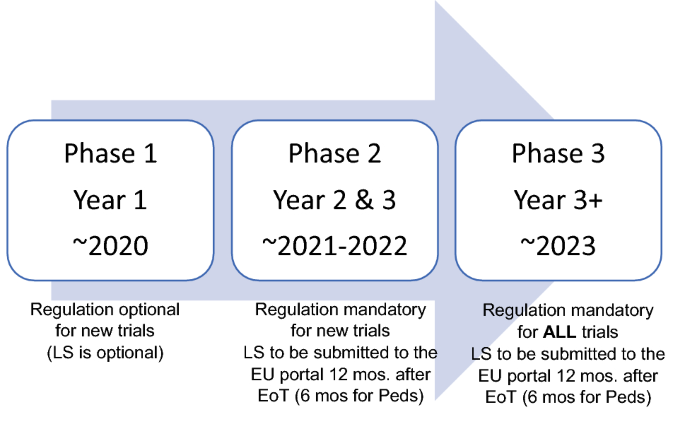
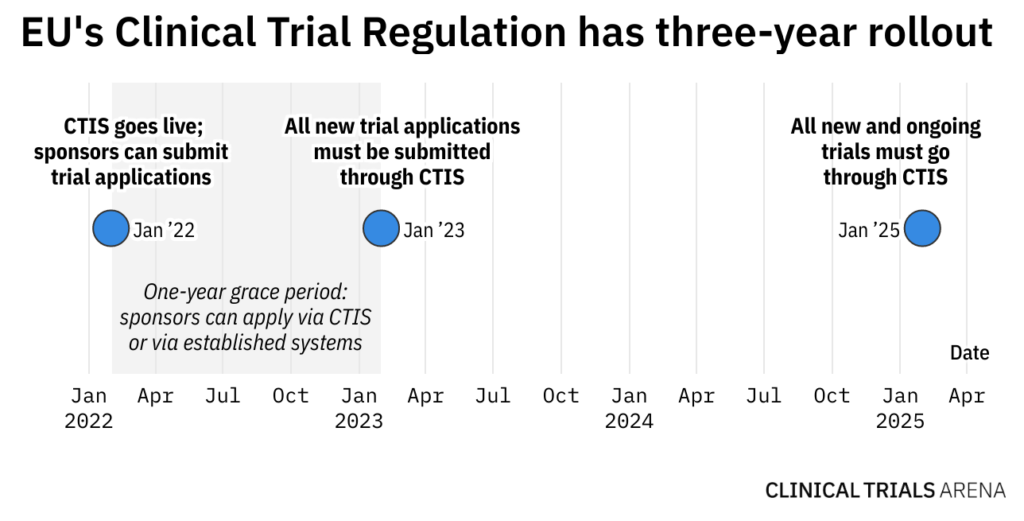
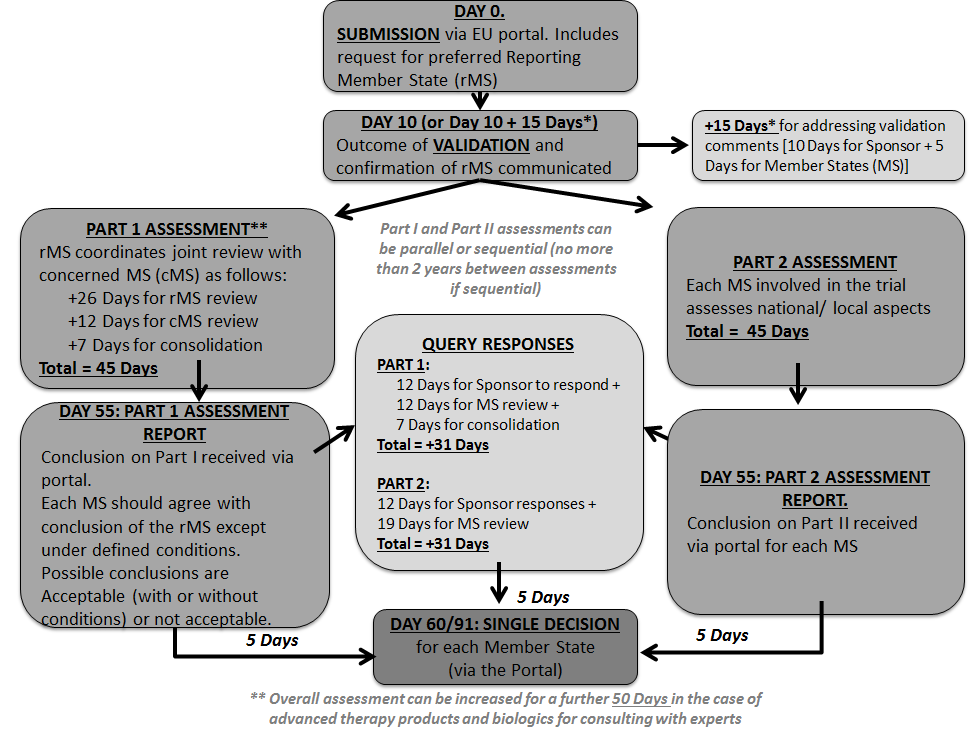
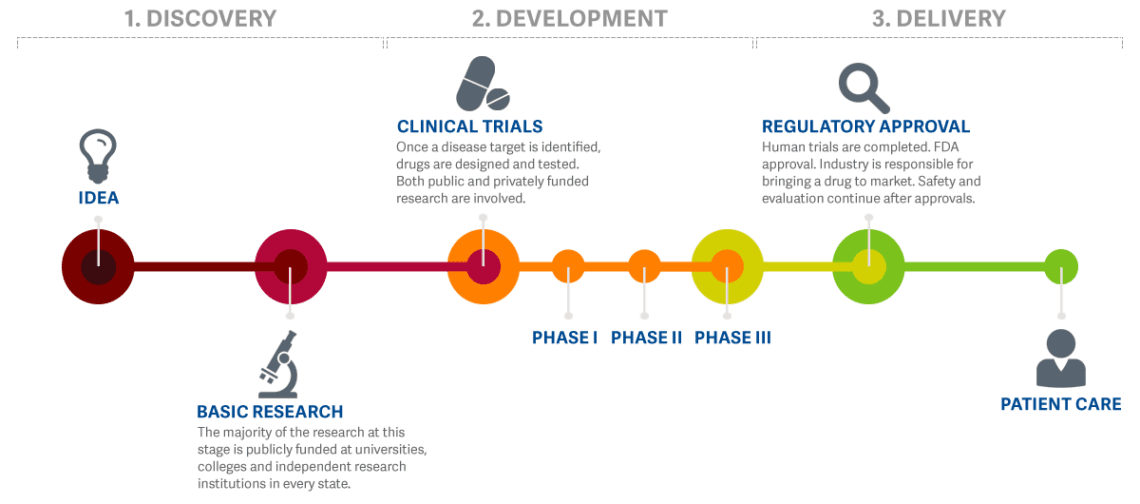
Managing the New EU Clinical Trials Regulation 536/2014 – Guidance for Navigating the Clinical Trial Information System (CTIS)